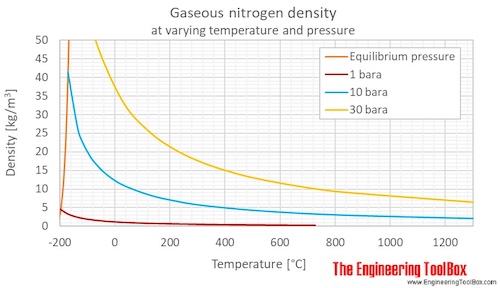
Web this page relies on the ideal gas law to calculate values of pressure at different temperatures: Molar mass, constant, temperature, pressure, volume, substance formula mkg/kmol rkj/kg·k* k mpa m3/kmol. N is the amount of gas, and r is the ideal gas constant. Nitrogen pressure (p), number of moles of nitrogen gas (n), ideal gas constant (r), and temperature (t) in kelvin. Values at 25 o c (77 o f, 298 k) and atmospheric pressure.
Pv = nrt, where p, v and t is the pressure, volume and temperature of gas respectively; Icemeister was curious as to how high pressures would be in his nitrogen cylinder when it was stored in a hot ares. Nitrogen pressure (p), number of moles of nitrogen gas (n), ideal gas constant (r), and temperature (t) in kelvin. Web enter the pressure and temperature in any of five units of pressure (atmospheres, bar, kilopascals, pounds per square inch, or millimeters of mercury) and five units of temperature (degrees celsius, kelvin, fahrenheit, rankine, or réaumur). This version uses nist refprop for much greater accuracy.
Consider a closed system, a tire for instance. His nitrogen pressure calculator used the ideal gas law to solve for final pressure. Values at 25 o c (77 o f, 298 k) and atmospheric pressure. Chemical, physical and thermal properties of nitrogen: Molar mass, constant, temperature, pressure, volume, substance formula mkg/kmol rkj/kg·k* k mpa m3/kmol.
Collect the values of three out of the four variables mentioned in the formula: Web enter the pressure and temperature in any of five units of pressure (atmospheres, bar, kilopascals, pounds per square inch, or millimeters of mercury) and five units of temperature (degrees celsius, kelvin, fahrenheit, rankine, or réaumur). Icemeister was curious as to how high pressures would be in his nitrogen cylinder when it was stored in a hot ares. Nitrogen pressure (p), number of moles of nitrogen gas (n), ideal gas constant (r), and temperature (t) in kelvin. Consider a closed system, a tire for instance. Web this page relies on the ideal gas law to calculate values of pressure at different temperatures: Web the phase diagram of nitrogen is shown below the table. Nitrogen n 2 28.013 0.2968 126.2 3.39 0.0899 nitrous oxide n Molar mass, constant, temperature, pressure, volume, substance formula mkg/kmol rkj/kg·k* k mpa m3/kmol. His nitrogen pressure calculator used the ideal gas law to solve for final pressure. N is the amount of gas, and r is the ideal gas constant. Pv = nrt, where p, v and t is the pressure, volume and temperature of gas respectively; Ensure that the units are consistent. This version uses nist refprop for much greater accuracy. Web calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve.
Web Calculation Of Thermodynamic State Variables Of Nitrogen In Saturation State, Boiling Curve.
Consider a closed system, a tire for instance. Ensure that the units are consistent. Icemeister was curious as to how high pressures would be in his nitrogen cylinder when it was stored in a hot ares. Collect the values of three out of the four variables mentioned in the formula:
Molar Mass, Constant, Temperature, Pressure, Volume, Substance Formula Mkg/Kmol Rkj/Kg·k* K Mpa M3/Kmol.
Nitrogen n 2 28.013 0.2968 126.2 3.39 0.0899 nitrous oxide n His nitrogen pressure calculator used the ideal gas law to solve for final pressure. This version uses nist refprop for much greater accuracy. Web the phase diagram of nitrogen is shown below the table.
N Is The Amount Of Gas, And R Is The Ideal Gas Constant.
Web enter the pressure and temperature in any of five units of pressure (atmospheres, bar, kilopascals, pounds per square inch, or millimeters of mercury) and five units of temperature (degrees celsius, kelvin, fahrenheit, rankine, or réaumur). Pv = nrt, where p, v and t is the pressure, volume and temperature of gas respectively; Chemical, physical and thermal properties of nitrogen: Values at 25 o c (77 o f, 298 k) and atmospheric pressure.
Nitrogen Pressure (P), Number Of Moles Of Nitrogen Gas (N), Ideal Gas Constant (R), And Temperature (T) In Kelvin.
Web this page relies on the ideal gas law to calculate values of pressure at different temperatures: