Web most of the values in this table are for data at a constant pressure of 760 torr. Pv = nrt, where p, v and t is the pressure, volume and temperature of gas respectively; As discussed in chapter 9, there should not be much difference between the constants obtained either way, which is observed for most of the pairs in this table for which both forms are shown. Web this page relies on the ideal gas law to calculate values of pressure at different temperatures: Web nitrogen pressure does not change with temp change, you have a leak, even if it changed 1lb you have a leak.
Web the following thermodynamic properties are calculated: Nitrogen n 2 28.013 0.2968 126.2 3.39 0.0899 nitrous oxide n Triple point temperature (crystal 2, crystal 1. Triple point temperature (crystal 1, liquid, and gas) 30 experimental data points. Web accurate thermophysical properties are available for several fluids.
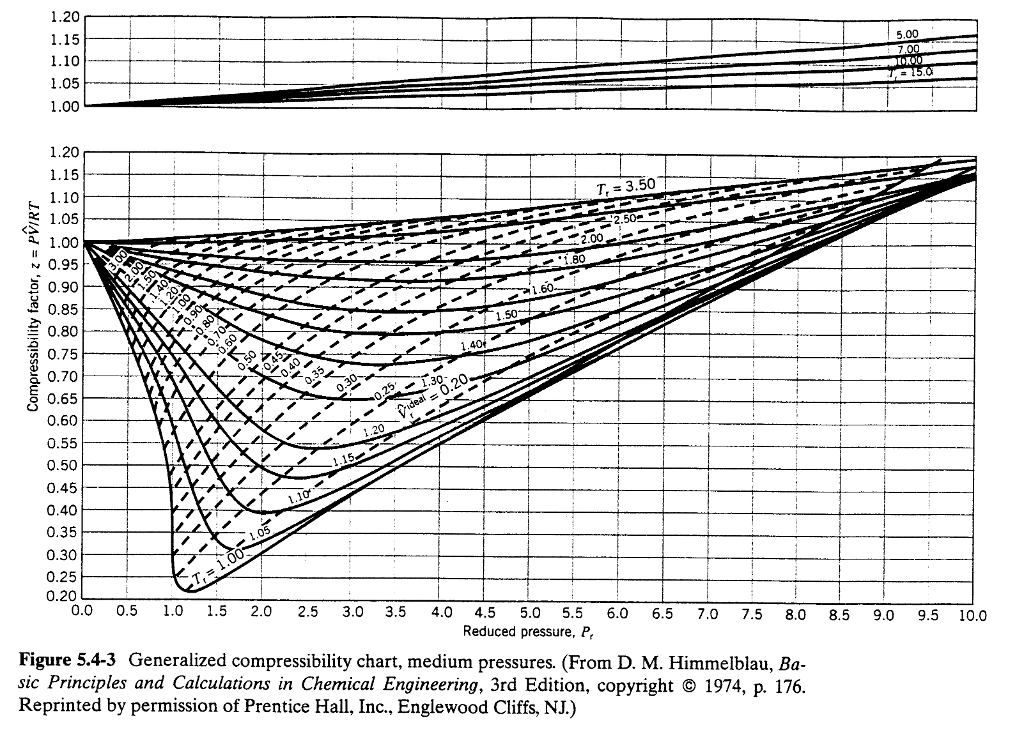
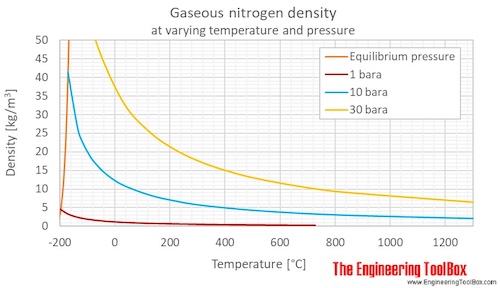
Web the reduced density and reduced temperature. Web this page relies on the ideal gas law to calculate values of pressure at different temperatures: Icemeister was curious as to how high pressures would be in his nitrogen cylinder when it was stored in a hot ares. Web nitrogen and methane viscosities are directly proportional to the pressure with a correlation coefficient of 4% and 97% respectively, and inversely proportional to temperature with a correlation. Molar mass, constant, temperature, pressure, volume, substance formula mkg/kmol rkj/kg·k* k mpa m3/kmol.
Otto, j., vapor pressure curves and triple points in the temperature region from 14 to 90 k, phys. The approximations are polynomials worked out by the least squares method. The only way the pressure changed is if there was another gas in the system. Web routines for the physical properties of nitrogen liquid and gas (1 to 6 bar) are presented. Web for this compound, wtt contains critically evaluated recommendations for: The fitted expressions have been compared with exper imental data, and the accuracy is. Nitrogen n 2 28.013 0.2968 126.2 3.39 0.0899 nitrous oxide n Web the following thermodynamic properties are calculated: His nitrogen pressure calculator used the ideal gas law to solve for final pressure. Web most of the values in this table are for data at a constant pressure of 760 torr. Triple point temperature (crystal 2, crystal 1. Web nitrogen and methane viscosities are directly proportional to the pressure with a correlation coefficient of 4% and 97% respectively, and inversely proportional to temperature with a correlation. Pv = nrt, where p, v and t is the pressure, volume and temperature of gas respectively; This version uses nist refprop for much greater accuracy. Web accurate thermophysical properties are available for several fluids.
Vapor Pressure Of Solid And Liquid.
Web accurate thermophysical properties are available for several fluids. Web the following thermodynamic properties are calculated: N is the amount of gas, and r is the ideal gas constant. The fitted expressions have been compared with exper imental data, and the accuracy is.
As Discussed In Chapter 9, There Should Not Be Much Difference Between The Constants Obtained Either Way, Which Is Observed For Most Of The Pairs In This Table For Which Both Forms Are Shown.
Web the reduced density and reduced temperature. Pv = nrt, where p, v and t is the pressure, volume and temperature of gas respectively; Nitrogen n 2 28.013 0.2968 126.2 3.39 0.0899 nitrous oxide n Web most of the values in this table are for data at a constant pressure of 760 torr.
The Model May Be Used To Calculate The Thermodynamic Properties Of Mixtures At Various Compositions Including Dew And
Web routines for the physical properties of nitrogen liquid and gas (1 to 6 bar) are presented. Giauque and clayton, 1933 giauque, w.f.; Clayton, j.o., heat capacity and entropy of nitrogen. Web nitrogen pressure does not change with temp change, you have a leak, even if it changed 1lb you have a leak.
Some Are Constant Temperatures, As Shown.
Triple point temperature (crystal 1, liquid, and gas) 30 experimental data points. Molar mass, constant, temperature, pressure, volume, substance formula mkg/kmol rkj/kg·k* k mpa m3/kmol. The temperature glide runs about 13°f in the evaporator. Otto, j., vapor pressure curves and triple points in the temperature region from 14 to 90 k, phys.